
The Company
flow-meter™ is active in the design and production of devices for measurement, control and supply of fluids, particularly for applications in the medical field. During the years, the great experience conquered in Italy and abroad created the conditions to establish strict partnerships with some of the most prestigious Groups operating in the industry of the medical gases worldwide. This made flow-meter™ as a recognized and respected “center of excellence” in this field. The company’s goal is headed to face with the complexity and the continuous technological evolution in the market of medical devices. This is possible only by investing in qualified human resources and developing related activities in design and technology. The strict respect of the medical field rules is absolute, receiving always from the Company management the maximum of attention.
History
flow-meter™ was established in 1969 by Franco Paratico. Originally, its products were principally destined to an industrial market and included standard processing instruments (flowmeters, flow switches, level switches, calibrated flanges) or specific instruments for industrial plants or machines. The experience gained in this sector brought the company to develop apparatus even for the medical sector. So doing, the range of products, be they standard or special, was widened and qualified technical resources were dedicated to improving and developing new technologies and services necessary to satisfy markets in continuous technological and normative evolution. Constant research, application and experimentation together with an international clientele and specialisation in medical and industrial sectors signifies a wealth of experience and proven scientific reputation.

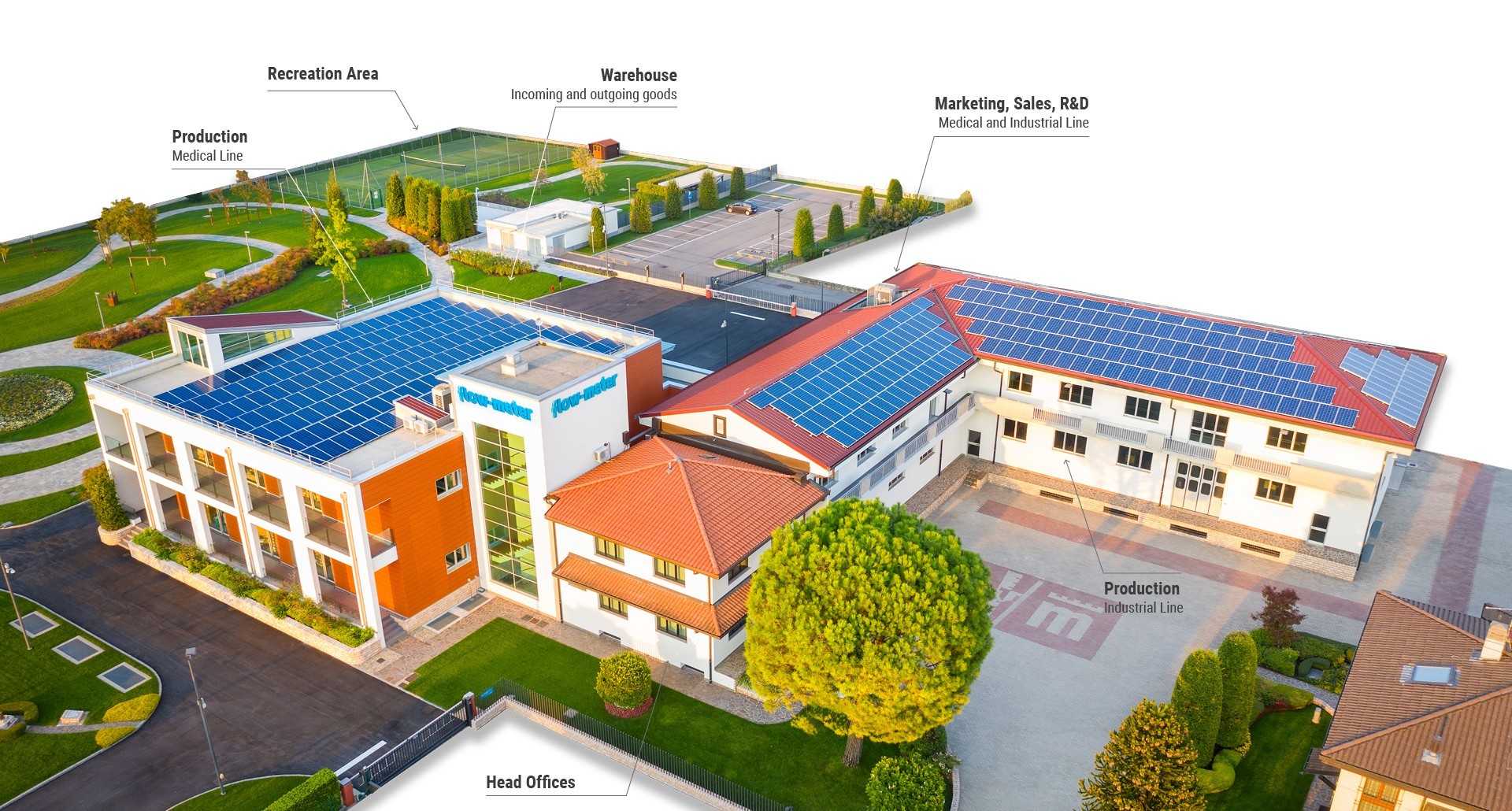
Production
The components, manufactured in-house or in outsourcing by qualified subcontractors, support first accurate controls, then pass to the assembly, always made inside the factory, and finally the complete devices are subjected to a rigorous testing procedure before being released on the market. Careful packaging and an efficient shipping system allow safe and quick delivery to customers and distributors worldwide. The production times for orders fulfillment are absolutely acceptable, in application of the operational flexibility, one of the peculiarity of Italian industry excellences, well known and recognized all over the world.
Quality
flow-meter™ based its corporate structure on a main factor of strategic importance: the Quality. In this regard, the Company has a Quality Assurance System certificated in accordance with:
UNI EN ISO 13485 : 2021 (Certificate No. 19026-M) issued by notified body Company KIWA Cermet S.p.A. |
All medical devices manufactured by flow-meter™ respect and comply the requirements of Regulation (EU) MDR 2017/745, and meet the technical specifications imposed by national and international reference standards. Before getting the official release to the production, then to the market, the developed new devices support rigorous tests, carried out both in the internal laboratory, and in qualified external centers, in order to guarantee the full compliance of all products to the foreseen conditions of use. For all medical devices manufactured by the Company, the process of CE marking is carried out by applying a complete quality system EN ISO 13485.
